Introduction
Materials and methods
Materials
Microwave continuous HDS device and process
Catalytic performance evaluation
Simulation of electromagnetic field and temperature distribution
Molecular simulation
Tab.1 Calculation parameters |
| Theory | DFT |
|---|---|
| Software | Materials Studio 6.1 |
| Module | Dmol3 |
| Functional | GGA/RPBE |
| Basis set | DNP |
| Density mixing charge | 0.03 |
| SCF tolerance | 1.0×10‒6 Ha |
| Convergence tolerance of energy | 1×10‒5 Ha |
| Convergence tolerance of Max. force | 0.002 Hatree/Å |
| Convergence tolerance of Max. displacement | 0.005 Å |
Reaction kinetics analysis
Results and discussion
Performance of microwave assisted HDS
Microwave thermal effect
Tab.2 HDS reaction rate constants at different temperatures |
| Temperature /°C | k (conventional heating) /s–1 | k (microwave heating) /s–1 | k (adjusted for microwave )/s–1 |
|---|---|---|---|
| 200 | 0.07 | 0.09 | 0.09 |
| 220 | 0.11 | 0.14 | 0.12 |
| 240 | 0.14 | 0.21 | 0.19 |
| 260 | 0.20 | 0.32 | 0.27 |
| 280 | 0.28 | 0.40 | 0.37 |
Microwave non-thermal effect
Analysis of apparent active energy and pre-exponential factor A
Tab.3 Pre-exponential factors and activation energy achieved |
| Factors | Conventional heating | Microwave heating |
|---|---|---|
| EA /(kJ·mol‒1) | 36.2 | 41.7 |
| A/s‒1 | 707 | 3633 |
Theoretical study of microwave enhanced HDS
Sulfur distribution of the thiophene model oils before and after the HDS process was investigated by gas chromatographic analysis. The results are shown in Table 4. Researchers commonly believe that thiophene HDS occurs via two parallel pathways: the DDS route to form intermediate products of butadiene and butenes and the HYD route to form intermediates of dihydrothiophene and tetrahydrothiophene. The intermediate 1-butanethiol is formed via both routes (Figs. 1 and 2) [19‒22]. However, there has been considerable debate regarding the mechanism of thiophene HDS. It has been difficult to establish to what extent prehydrogenation to dihydrothiophene or tetrahydrothiophene may be necessary before S‒C bond scission [20,38,39]. From Table 4, the contents of thiophene and total sulfur via microwave HDS were lower than those from conventional HDS at the same temperature, indicating that microwave can highly enhance the HDS reaction. At the same conditions, the HDS activities of sulfur compounds decrease in the order of mercaptan, tetrahydrothiophene and thiophene. However, from thermodynamic analysis, at low temperature (such as 220°C), the equilibrium constant of thiophene is much higher than those of 1-butanethiol and tetrahydrothiophene [40]. This could explain the tetrahydrothiophene and 1-butanethiol detected in the products, even at high temperature of 280°C.
Tab.4 Sulfur distribution of raw FCC model oil and HDS productsa) |
| Temperature /°C | CS2/ppm | Thiophene /ppm | 1-Butanethiol /ppm | Tetrahydrothiophene /ppm | Total sulfur /ppm | Thiophene removal /% |
|---|---|---|---|---|---|---|
| Raw material | 3.65 | 277.49 | 0 | 0 | 281.14 | |
| 200 MW | 7.66 | 116.78 | 0.68 | 3.58 | 128.7 | 57.92 |
| 220 MW | 16.04 | 58.52 | 0.43 | 1.59 | 76.58 | 78.91 |
| 240 MW | 11.21 | 28.7 | 0.28 | 0.63 | 40.82 | 89.66 |
| 260 MW | 4.95 | 10.79 | 0.10 | 0.46 | 16.3 | 96.11 |
| 280 MW | 1.54 | 5.42 | 0.22 | 0.90 | 8.08 | 98.05 |
| 200 | 5.67 | 140.29 | 0.73 | 1.28 | 147.97 | 49.44 |
| 220 | 17.49 | 88.91 | 0.48 | 1.19 | 108.07 | 67.96 |
| 240 | 14.11 | 63.4 | 0.59 | 1.87 | 79.97 | 77.15 |
| 260 | 5.27 | 42.04 | 0.29 | 0.61 | 48.21 | 84.85 |
| 280 | 4.22 | 18.57 | 0.15 | 0.57 | 23.51 | 93.31 |
a) MW: via microwave treatment. Error: ±1%. |
Under the HDS conditions, H2 strips sulfur from the catalyst, makes it partly reduced, and starts to crack hydrocarbons by hydrogenolysis. CH4 will be formed, reacting with H2S to CS2. The formation of CS2, thus, has nothing to do with the DDS or HYD mechanisms. As shown in Table 4, the contents of tetrahydrothiophene were relatively higher through microwave treatment than those from conventional heating, whilst the contents of 1-butanethiol were lower under microwave irradiation. As from Figs. 1 and 2, both reaction routes can form 1-butanethiol, whereas tetrahydrothiophene can only be formed through HYD pathway, therefore one can conclude that the HDS process tends to proceed via HYD route under microwave conditions. Compared to the hydrocarbon content, the thiophene content was quite low, and the produced butadiene and butene are easily saturated, thus these compounds were not detected.
The microwave heating effect arises from the interaction between the electric field and the charged molecules in the material. The power absorbed by a material is dependent on the dielectric properties of the material. The absorbed power density (Pd) (W∙m‒3), is given by [41]:
where f is microwave frequency (Hz); e0 is the dielectric permittivity of free space, 8.85×10‒12 F∙m‒1; E is the electric field strength inside the material (V∙m‒1); e" is the dielectric loss factor. Equation (15) states that the power absorbed varies linearly with the loss factor, i.e., a high loss material will be heated more rapidly than a low loss material. In general, the higher the induced polarity, the greater the influence that microwaves may induce. The polarities of all components present in the reaction medium play an important role in the microwave heating rate. Thus, the dipole moment was investigated through Materials Studio, and the results are listed in Table 5. The higher the dipole moment, the higher the dielectric properties of the material are. Therefore, the polar sulfur compounds within the FCC gasoline model oil would readily interact with the microwave electromagnetic field and absorb more microwave energy (Eq. (15)). Carbon disulfide, a non-polar material formed during HDS reaction, is difficult to treat by microwave, hindering its continuous reaction. Thus, there would be a higher content of CS2 in the reaction system under microwave irradiation than with conventional heating.
Tab.5 Dipole moment values achieved from MS Dmol3 modular |
| Material | Thiophene | Tetrahydrothiophene | 2,3-Dihydro-thiophene | 1-Butanethiol | Butadiene | CS2 |
|---|---|---|---|---|---|---|
| m /debye | 0.3679 | 1.9588 | 1.4573 | 1.6724 | 0.0022 | 0.0006 |
The bond order is a physical parameter describing the bond strength between adjacent atoms in a molecule. The higher the bond order, the stronger the bond will be. As shown in Fig. 10, the bond order of the C‒S bond was reduced from 1.289 in thiophene to 1.044 in tetrahydrothiophene. It indicates that the C‒S bond is weakened after hydrogenation and is more prone to dissociation. The intermediate products of 2,3-dihydrothiophene and tetrehydrothiophene from the HYD pathway have certain polarity (Table 6 and Fig. 10) and are easily heated by microwave. Therefore, once 2,3-dihydrothiophene, tetrehydrothiophene are generated, they will continue to react immediately under microwave irradiation to produce the final product. This theory agrees with that of Rodríguez et al. [10], who stated that if the relative polarity increases from the reactant to the transition structure, the acceleration due to an increase in microwave absorbance of the intermediate could occur. This effect is particularly important in produce-like transition states according to Hammond’s postulate [10,42].
Tab.6 Pre-exponential factors and activation energy achieved |
| Factors | Conventional heating | Microwave heating |
|---|---|---|
| EA /(kJ·mol‒1) | 29.1 | 33.0 |
| A /s‒1 | 166 | 465 |
The mechanism of HDS is highly dependent on the interaction between sulfides and catalysts. Thiophene adsorption is the first step in the HDS catalytic process [23]. Studies have shown that thiophene is weakly chemisorbed to the catalyst surface in h5 geometry (parallel to the surface) and h1 (S) geometry (upright S-bind) [19,24,43‒45]. Wiegand et al. [24] reported that the orientation of thiophene with respect to metal surfaces is sensitive to its coverage and the coverage of co-adsorbed hydrocarbons and sulfur. On most surfaces, a parallel geometry is favored at low coverage and perpendicular inclination of thiophene ring is favored at high coverage. These coverage-dependent phenomena are probably due to the intermolecular interactions of sulfur components with catalysts, and also related to the steric interactions with neighboring ligands in a discrete cluster or complex. Borges et al. [18] found that thiophene coordinates with two MoIV atoms through a distorted bridge-h5 bonding, even if the optimization process started from a h1 geometry, and the results is consistent with other researchers [25,26]. After adsorption the lengths of C‒S bond, the C=C double bonds increased by 0.1 and 0.05Å respectively. From the electronic analysis, a donation of thiophene p electrons to the catalyst surface through Mo1 and a back charge donation through Mo2 of the catalyst to S of the absorbed thiophene molecule were also observed [18,23,26]. The bridge-h5 type thiophene configuration indicates that the aromatic character of the thiophene ring was destroyed by the adsorption. This means that an adsorption geometry is the key factor to weaken the C‒S bond and to reduce the aromatic character of the thiophene ring, i.e., making the molecule more prone to both desulfurization and hydrogenation [25].
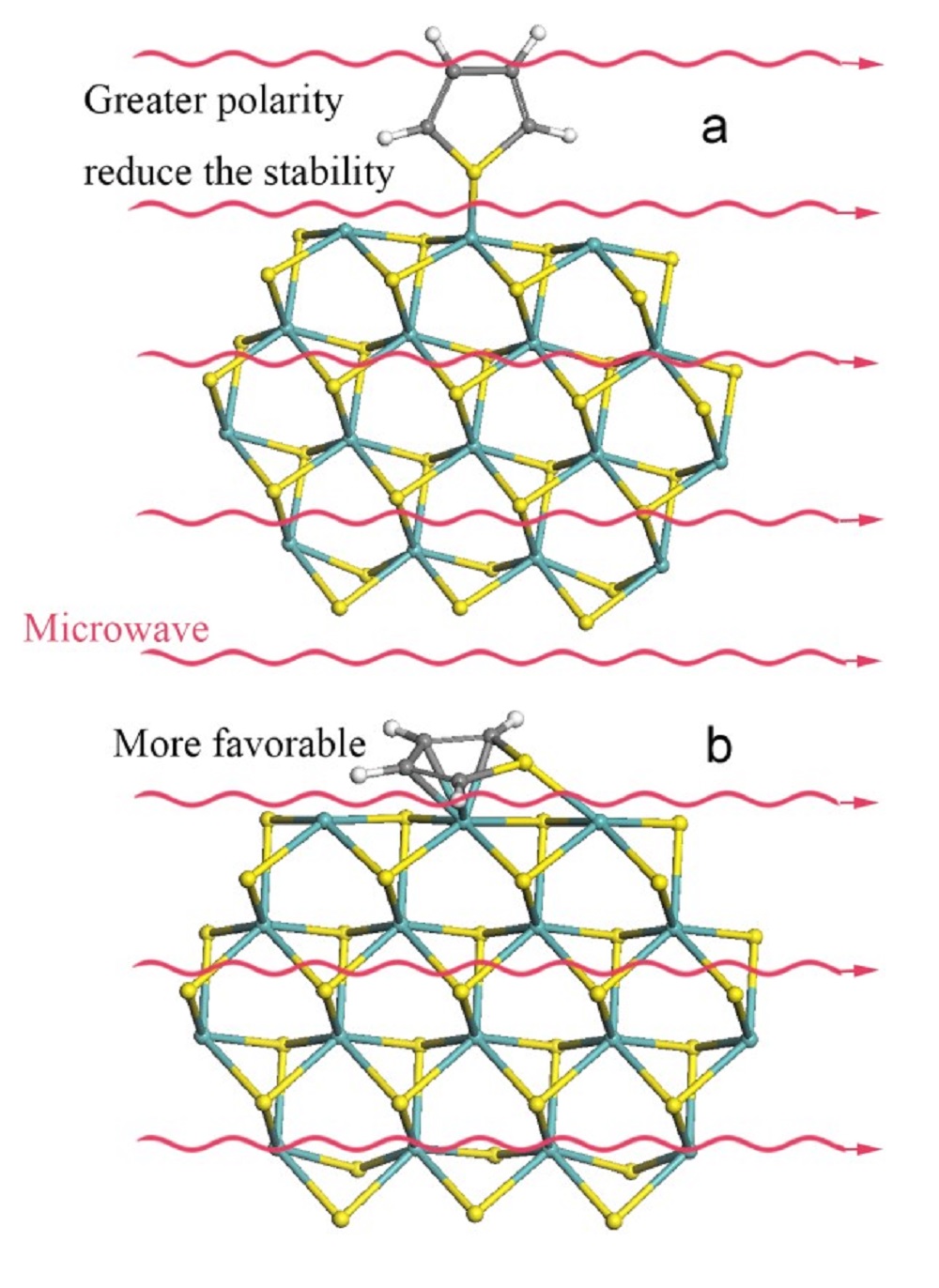
It was suggested that the cleavage of the C–S bond occurs when thiophene is adsorbed on a coordinately unsaturated Mo site via the sulfur atom in the s mode [19], i.e., the DDS pathway is more likely to occur through s adsorption of thiophene molecule via sulfur atom (h1 (S)), and the HYD pathway proceeds through p adsorption (h5) of the reactant via the aromatic system. The interaction of thiophene with molybdenum clusters from the calculations modeling show that the lengths of C=C and C‒S bonds increased after adsorption through h5 [18,46], making thiophene molecules more polar, thus would absorb more microwave energy, i.e., microwave would further enhance the HDS process. Therefore, under microwave irradiation, HDS favors the hydrogenation route. However, the S-M (sulfur-metal) bond formed from s adsorption (h1 (S)) would make the structure more polar, hindering the adsorption and decreasing the stability of the S-M structure in a strong electromagnetic field (Fig. 11). For p (h5) adsorption, since the thiophene molecular absorbed is parallel to the catalyst surface, which in theory is little influenced by microwave field. Based on the above discussions, after h5 adsorption, the structure of thiophene becomes more polar, therefore microwave will highly enhance the reaction through this route.

Fig.11 Adsorption of thiophene through (a) h1 and (b) h5 modes on an MoS2 cluster model catalyst. |
The sulfur coverage was high at the beginning of the HDS process. In this case the adsorption was mainly through h1 (S), which would be hindered by microwave electromagnetic field as discussed above. This could explain the higher apparent activation energy for microwave heating compared to conventional heating. To verify this conclusion, a series of HDS experiments were carried out using a lower thiophene content model oil as the raw material under microwave and conventional heating methods. These experiments were designed to investigate whether the adsorption mode would change at different initial sulfur coverage. The thiophene content was 203.58 ppm for the experiments. Microwave assisted HDS reactions were employed at a pressure of 2 MPa, WHSV of 2 h‒1, hydrogen/oil ratio of 200:1, and temperatures of 240°C, 250°C, 260°C and 270°C. The same temperatures were used for the HDS reactions with conventional heating. The desulfurization results and the constant reaction rates calculated based on Eq. (11) at various temperatures are shown in Table 7. The apparent activation energy and pre-exponential factor can be achieved based on Fig. 12 and Eq. (12).
Tab.7 HDS ratio and reaction rate constants at different temperatures |
| Temperature /°C | HDS ratio (conventional heating) /% | HDS ratio (microwave heating) /% | k (conventional heating) /s–1 | k (microwave heating) /s–1 |
|---|---|---|---|---|
| 240 | 81.06 | 84.73 | 0.18 | 0.21 |
| 250 | 84.50 | 87.66 | 0.21 | 0.23 |
| 260 | 87.77 | 91.64 | 0.23 | 0.28 |
| 270 | 90.46 | 94.12 | 0.26 | 0.32 |
| 280 | 93.58 | 96.11 | 0.30 | 0.36 |

Fig.12 Linear regression of lnk versus 1000/RT under conventional and microwave HDS. |
Compared with the results from Table 2, when the contents of thiophene decreased from 277.49 to 203.58 ppm, the apparent activation energy decreased. The difference in the activation energy between conventional heating and microwave assisted HDS was also significantly reduced. These results highly support the above theory that the upright S-bind geometry under microwave is less stable in the microwave electromagnetic field. However, the parallel adsorption will be strengthened, and microwave electromagnetic field would enhance h5 adsorption and the HDS process would mainly follow the hydrogenation route.