

c-Fos expression in rat brainstem following intake of sucrose or saccharin
Received date: 22 Jan 2011
Accepted date: 10 Jun 2011
Published date: 05 Sep 2011
Copyright
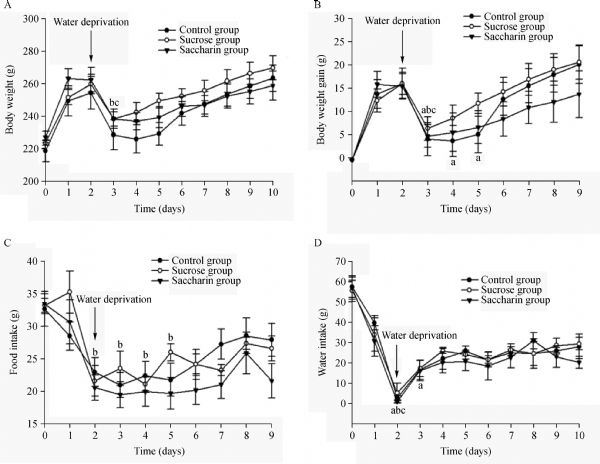
To examine whether the activation of brainstem neurons during intake of a sweet tastant is due to orosensory signals or post-ingestive factors, we compared the distribution of c-Fos-like immunoreactivity (c-FLI) in the nucleus of the solitary tract (NST) and parabrachial nucleus (PBN) of brainstem following ingestion of 0.25 M sucrose or 0.005 M saccharin solutions. Immunopositive neurons were localized mainly in the middle zone of the PBN and four rostral-caudal subregions of the NST. Intake of sucrose increased the number of FLI neurons in almost every subnucleus of the PBN (F(2,13) = 7.610, P = 0.023), in addition to the caudal NST at the level of the area postrema (F(2,13) = 10.777, P = 0.003) and the NST intermediate zone (F(2,13) = 7.193, P = 0.014). No significant increase in the number of c-Fos positive neurons was detected in response to saccharin ingestion, although there was a trend towards a modest increase in a few select NST and PBN nuclei. These results suggest that the PBN and NST may be involved in sweet taste perception and modulation of sweet tastant intake, but the significantly enhanced intensity of Fos expression induced by sucrose indicates that PBN/NST neuronal activity is driven by the integrated effects of sweet taste sensation and post-ingestive signals.
Key words: c-Fos; parabrachial; the nucleus of the solitary tract; sweet tastant; rat
Ke Chen , Jianqun Yan , Jinrong Li , Bo Lv , Xiaolin Zhao . c-Fos expression in rat brainstem following intake of sucrose or saccharin[J]. Frontiers of Medicine, 2011 , 5(3) : 294 -301 . DOI: 10.1007/s11684-011-0144-8
| 1 |
Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science 1988; 240(4857): 1328-1331
|
| 2 |
Harrer MI, Travers SP. Topographic organization of Fos-like immunoreactivity in the rostral nucleus of the solitary tract evoked by gustatory stimulation with sucrose and quinine. Brain Res 1996; 711(1-2): 125-137
|
| 3 |
King CT, Travers SP, Rowland NE, Garcea M, Spector AC. Glossopharyngeal nerve transection eliminates quinine-stimulated fos-like immunoreactivity in the nucleus of the solitary tract: implications for a functional topography of gustatory nerve input in rats. J Neurosci 1999; 19(8): 3107-3121
|
| 4 |
Travers JB, Urbanek K, Grill HJ. Fos-like immunoreactivity in the brain stem following oral quinine stimulation in decerebrate rats. Am J Physiol 1999; 277(2 Pt 2): R384-R394
|
| 5 |
Yamamoto T, Shimura T, Sako N, Sakai N, Tanimizu T, Wakisaka S. c-Fos expression in the parabrachial nucleus after ingestion of sodium chloride in the rat. Neuroreport 1993; 4(11): 1223-1226
|
| 6 |
Yamamoto T, Shimura T, Sakai N, Ozaki N. Representation of hedonics and quality of taste stimuli in the parabrachial nucleus of the rat. Physiol Behav 1994; 56(6): 1197-1202
|
| 7 |
Yamamoto T, Sako N, Sakai N, Iwafune A. Gustatory and visceral inputs to the amygdala of the rat: conditioned taste aversion and induction of c-fos-like immunoreactivity. Neurosci Lett 1997; 226(2): 127-130
|
| 8 |
Yamamoto T, Sawa K. c-Fos-like immunoreactivity in the brainstem following gastric loads of various chemical solutions in rats. Brain Res 2000; 866(1-2): 135-143
|
| 9 |
Kobashi M, Ichikawa H, Sugimoto T, Adachi A. Response of neurons in the solitary tract nucleus, area postrema and lateral parabrachial nucleus to gastric load of hypertonic saline. Neurosci Lett 1993; 158(1): 47-50
|
| 10 |
Ågmo A, Marroquin E. Role of gustatory and postingestive actions of sweeteners in the generation of positive affect as evaluated by place preference conditioning. Appetite 1997; 29(3): 269-289
|
| 11 |
Kushner LR, Mook DG. Behavioral correlates of oral and postingestive satiety in the rat. Physiol Behav 1984; 33(5): 713-718
|
| 12 |
Yamamoto T, Sawa K. Comparison of c-fos-like immunoreactivity in the brainstem following intraoral and intragastric infusions of chemical solutions in rats. Brain Res 2000; 866(1-2): 144-151
|
| 13 |
Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res 1984;7(3):229-259
|
| 14 |
Travers SP , Hu H. Extranuclear projections of rNST neurons expressing gustatory-elicited Fos. J Comp Neurol2000; 427(1): 124-138
|
| 15 |
Chen K, Yan J, Suo Y, Li J, Wang Q, Lv B.Nutritional status alters saccharin intake and sweet receptor mRNA expression in rat taste buds. Brain Res 2010; 1325:53-62
|
| 16 |
Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA 2007; 104(38): 15075-15080
|
| 17 |
Nakagawa Y, Nagasawa M, Yamada S, Hara A, Mogami H, Nikolaev VO, Lohse MJ, Shigemura N, Ninomiya Y, Kojima I.Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS ONE 2009; 4(4): e5106
|
| 18 |
Mungarndee SS, Lundy RF Jr, Norgren R. Expression of Fos during sham sucrose intake in rats with central gustatory lesions. Am J Physiol Regul Integr Comp Physiol 2008; 295(3): R751-R763
|
| 19 |
Hamilton RB, Norgren R. Central projections of gustatory nerves in the rat. J Comp Neurol 1984; 222(4): 560-577
|
| 20 |
Halsell CB, Travers SP, Travers JB. Ascending and descending projections from the rostral nucleus of the solitary tract originate from separate neuronal populations. Neuroscience 1996; 72(1): 185-197
|
| 21 |
Travers JB. Efferent projections from the anterior nucleus of the solitary tract of the hamster. Brain Res 1988; 457(1): 1-11
|
| 22 |
Halsell CB, Travers SP. Anterior and posterior oral cavity responsive neurons are differentially distributed among parabrachial subnuclei in rat. J Neurophysiol 1997; 78(2): 920-938
|
| 23 |
Karimnamazi H, Travers SP, Travers JB. Oral and gastric input to the parabrachial nucleus of the rat. Brain Res 2002; 957(2): 193-206
|
| 24 |
Yamamoto T. Brain mechanisms of sweetness and palatability of sugars. Nutr Rev 2003; 61(Supplement s5): S5-S9
|
| 25 |
Streefland C, Farkas E, Maes FW, Bohus B. c-fos expression in the brainstem after voluntary ingestion of sucrose in the rat. Neurobiology (Bp) 1996; 4(1-2): 85-102
|
| 26 |
Travers SP, Norgren R. Organization of orosensory responses in the nucleus of the solitary tract of rat. J Neurophysiol 1995; 73(6): 2144-2162
|
| 27 |
Whitehead MC, Frank ME. Anatomy of the gustatory system in the hamster: central projections of the chorda tympani and the lingual nerve. J Comp Neurol 1983; 220(4): 378-395
|
| 28 |
Schwarz J, Burguet J, Rampin O, Fromentin G, Andrey P, Tomé D, Maurin Y, Darcel N. Three-dimensional macronutrient-associated Fos expression patterns in the mouse brainstem. PLoS ONE 2010; 5(2): e8974
|
| 29 |
Norgren R, Pfaffmann C. The pontine taste area in the rat. Brain Res 1975; 91(1): 99-117
|
| 30 |
Tokita K, Shimura T, Nakamura S, Inoue T, Yamamoto T. Involvement of forebrain in parabrachial neuronal activation induced by aversively conditioned taste stimuli in the rat. Brain Res 2007; 1141:188-196
|
| 31 |
Geran LC, Travers SP. Bitter-responsive gustatory neurons in the rat parabrachial nucleus. J Neurophysiol 2009; 101(3): 1598-1612
|
| 32 |
Yamamoto T. Neural substrates for the processing of cognitive and affective aspects of taste in the brain. Arch Histol Cytol 2006; 69(4): 243-255
|
| 33 |
Norgren R, Smith GP. Central distribution of subdiaphragmatic vagal branches in the rat. J Comp Neurol 1988; 273(2): 207-223
|
| 34 |
Sakai N, Yamamoto T. Conditioned taste aversion and c-fos expression in the rat brainstem after administration of various USs. Neuroreport 1997; 8(9-10): 2215-2220
|
/
| 〈 |
|
〉 |