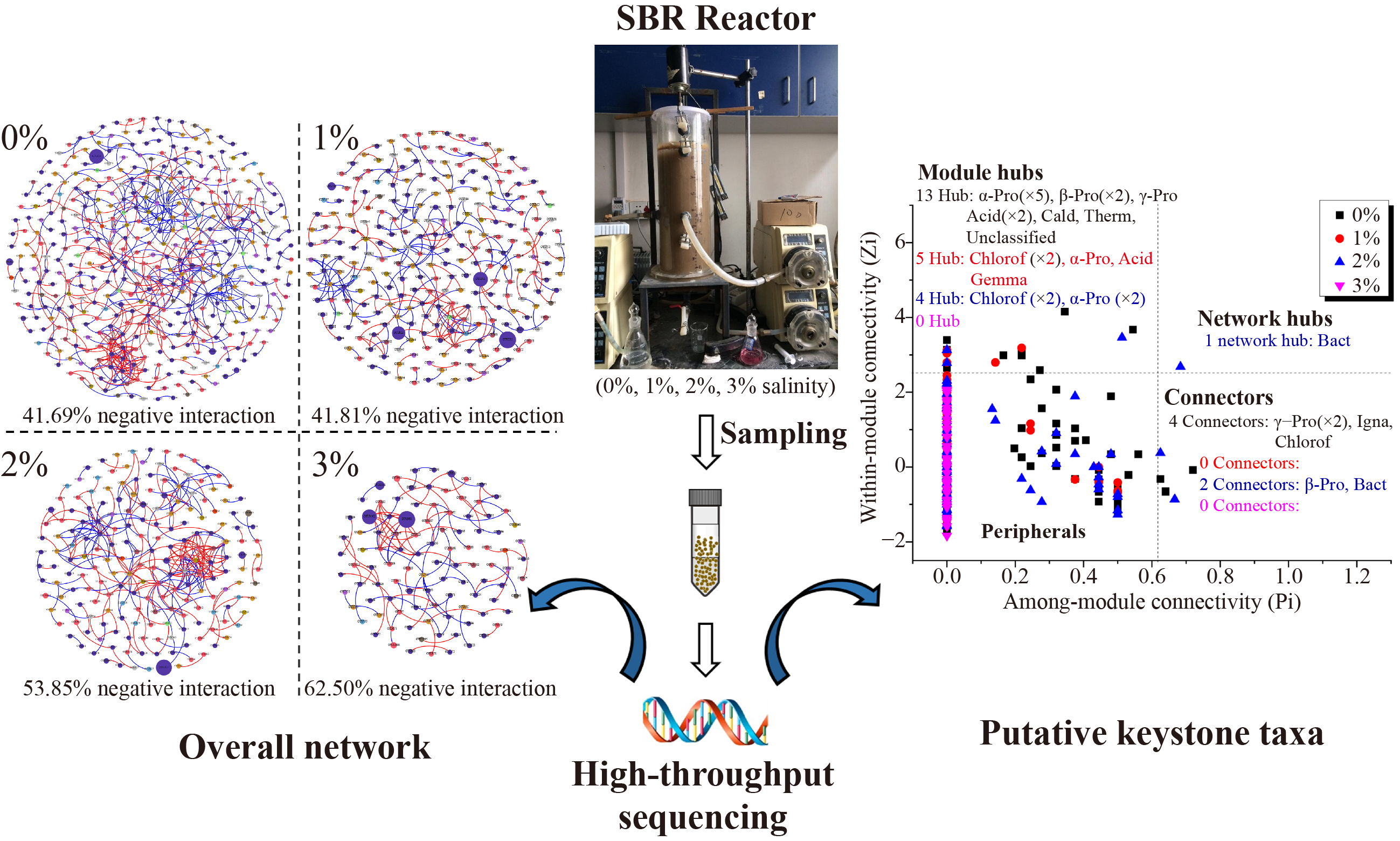
Some denitrifiers, such as
Acinetobacter,
Thauera,
Azoarcus,
Paracoccus,
Denitromonas, and
Defluviicoccus, were also used to construct subnetworks (Fig.5). The size and complexity of subnetworks of
Acinetobacter and
Thauera were both declined by elevating salinity. For example, the four
Acinetobacter subnetworks contained 17, 14, 12, and 8 nodes with 14, 11, 9, and 5 links, respectively, along with a salinity gradient. Besides, the elevated salinity stress decreased the abundance of
Acinetobacter (from 2.10% to 0.06%–0.61%), whereas enhanced
Thauera abundance (from 1.70% to 2.66%–11.18%). Although a similar trend of these two subnetworks was observed, the abundance trends of
Acinetobacter and
Thauera were different. This inconsistency highlighted the necessity of studying the bacterial interaction, as it provided a pivotal dimension to understand the microbial assembly process rather than the simple community richness and diversity. In addition, the most complex subnetwork of
Azoarcus appeared at 2% salinity, and the most complex subnetwork of
Paracoccus appeared at 3% salinity, while the most complex subnetwork of
Denitromonas appeared at 0% salinity.
Defluviicoccus, an endogenous denitrifier, was found dominant at 0%–2% salinity (relative abundance: 37.15%, 52.07%, 35.01%, and 8.80% according to 0%–3% salinity) under this intermittent aeration sewage treatment system (
Wang et al., 2020b). However, it appeared that 1%–3% salinity seriously reduced the microbial interactions in
Defluviicoccus subnetworks, as the subnetworks contained 74, 32, 44, and 14 nodes with 63, 25, 41, and 10 links, respectively (Fig. S4). It is worth noting that the percentage of negative relationships in subnetworks of
Acinetobacter,
Thauera,
Azoarcus,
Paracoccus, and
Denitromonas reduced from 92.86%, 88.24%, 75.00%, 100%, and 87.50% to 40%, 50%, 71.43%, 33.33%, and 84.62%, respectively, according to 0% and 3% salinities, indicating denitrifiers established more proportion of cooperative relationships with other bacteria to resist 3% salinity stress. The above results indicated that elevated salinity could have different influences on the microbial interactions of various bacteria with the same function to ensure the stability of the activated sludge system, even at 3% salinity.