

Frontiers of Chemical Science and Engineering >
A combination process of mineral carbonation with SO2 disposal for simulated flue gas by magnesia-added seawater
Received date: 16 Jan 2019
Accepted date: 27 May 2019
Published date: 15 Dec 2019
Copyright
The desulfurization by seawater and mineral carbonation have been paid more and more attention. In this study, the feasibility of magnesia and seawater for the integrated disposal of SO2 and CO2 in the simulated flue gas was investigated. The process was conducted by adding MgO in seawater to reinforce the absorption of SO2 and facilitate the mineralization of CO2 by calcium ions. The influences of various factors, including digestion time of magnesia, reaction temperature, and salinity were also investigated. The results show that the reaction temperature can effectively improve the carbonation reaction. After combing SO2 removal process with mineral carbonation, Ca2+ removal rate has a certain degree of decrease. The best carbonation condition is to use 1.5 times artificial seawater (the concentrations of reagents are 1.5 times of seawater) at 80°C and without digestion of magnesia. The desulfurization rate is close to 100% under any condition investigated, indicating that the seawater has a sufficient desulfurization capacity with adding magnesia. This work has demonstrated that a combination of the absorption of SO2 with the absorption and mineralization of CO2 is feasible.
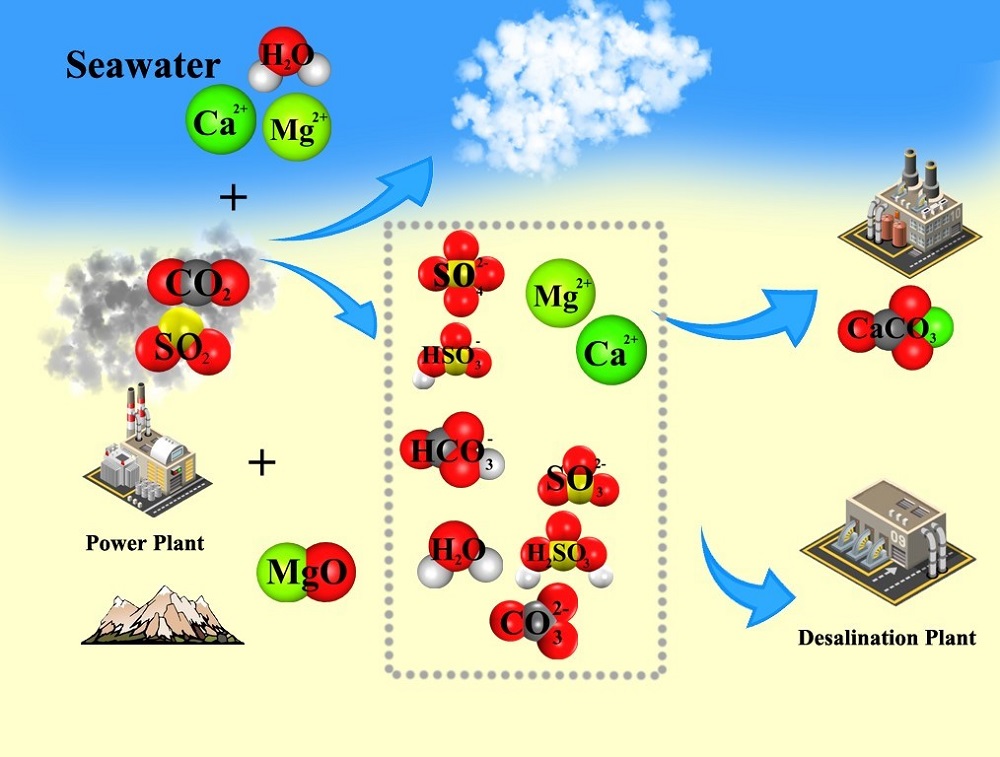
Key words: mineral carbonation; wet SO2 disposal; seawater; desulfurization
Yingying Zhao , Mengfan Wu , Zhiyong Ji , Yuanyuan Wang , Jiale Li , Jianlu Liu , Junsheng Yuan . A combination process of mineral carbonation with SO2 disposal for simulated flue gas by magnesia-added seawater[J]. Frontiers of Chemical Science and Engineering, 2019 , 13(4) : 832 -844 . DOI: 10.1007/s11705-019-1871-7
| 1 |
World Meteorological Organization: The state of greenhouse gases in the atmosphere based on global observations through 2016. WMO Greenhouse Gas Bulletin (GHG Bulletin), 2017-10-30
|
| 2 |
UN News Centre. Carbon dioxide levels surge to new high in 2016. UN weather agency reports, 2017-10-30
|
| 3 |
Aslam A, Thomas-Hall S R, Mughal T A, Schenk P M. Selection and adaptation of microalgae to growth in 100% unfiltered coal-fired flue gas. Bioresource Technology, 2017, 233: 271–283
|
| 4 |
Yadav G, Karemore A, Dash S K, Sen R. Performance evaluation of a green process for microalgal CO2 sequestration in closed photobioreactor using flue gas generated in-situ. Bioresource Technology, 2015, 191: 399–406
|
| 5 |
Liémans I, Thomas D. Simultaneous NOx and SOx Reduction from oxyfuel exhaust gases using acidic solutions containing hydrogen peroxide. Energy Procedia, 2013, 37: 1348–1356
|
| 6 |
Mumford K A, Yue W, Smith K H, Stevens G W. Review of solvent based carbon-dioxide capture technologies. Frontiers of Chemical Science and Engineering, 2015, 9(2): 125–141
|
| 7 |
Metz B, Davidson O, Coninck H C, Loos M, Meyer L A. Carbon Dioxide Capture and Storage. New York: Cambridge University Press, 2005, 5: 197–265
|
| 8 |
Gaus I. Role and impact of CO2—rock interactions during CO2, storage in sedimentary rocks. International Journal of Greenhouse Gas Control, 2010, 4(1): 73–89
|
| 9 |
Baines S J, Worden R H. The long-term fate of CO2 in the subsurface: Natural analogues for CO2 storage. Geological Society of London, Special Publications, 2004, 233(1): 59–85
|
| 10 |
Gunter W D, Perkins E H, Hutcheon I. Aquifer disposal of acid gases: Modelling of water—rock reactions for trapping of acid wastes. Applied Geochemistry, 2000, 15(8): 1085–1095
|
| 11 |
Xu R, Li R, Ma J, He D, Jiang P. Effect of mineral dissolution/precipitation and CO2 exsolution on CO2 transport in geological carbon storage. Accounts of Chemical Research, 2017, 50(9): 2056–2066
|
| 12 |
Park S, Lee M G, Park J. CO2 (carbon dioxide) fixation by applying new chemical absorption-precipitation methods. Energy, 2013, 59(11): 737–742
|
| 13 |
Liang Y, Tsuji S, Jia J, Tsuji T, Matsuoka T. Modeling CO2-water-mineral wettability and mineralization for carbon geosequestration. Accounts of Chemical Research, 2017, 50(7): 1530–1540
|
| 14 |
Teng H, Masutani S, Kinoshita C, Nihous G. Solubility of CO2 in the ocean and its effect on CO2 dissolution. Fuel and Energy Abstracts, 1996, 37(4): 308
|
| 15 |
Weiss R F. Carbon dioxide in water and seawater: The solubility of a non-ideal gas. Marine Chemistry, 1974, 2(3): 203–215
|
| 16 |
Zhao Y, Yuan J, Zhang J, Xie L, Ji Z, Su M, Chen J. A different approach for seawater decalcification pretreatment using carbon dioxide as precipitator. Desalination, 2013, 322(4): 151–158
|
| 17 |
Zhao Y, Cao H, Xie Y, Yuan J, Ji Z, Yan Z. Mechanism studies of a CO2 participant softening pretreatment process for seawater desalination. Desalination, 2016, 393: 166–173
|
| 18 |
Zhao Y, Zhang Y, Liu J, Gao J, Ji Z, Guo X, Liu J, Yuan J. Trash to treasure: Seawater pretreatment by CO2 mineral carbonation using brine pretreatment waste of soda ash plant as alkali source. Desalination, 2017, 407: 85–92
|
| 19 |
Gao J, Wang S, Zhao B, Qi G, Chen C. Pilot-scale experimental study on the CO2 capture process with existing of SO2: Degradation, reaction rate, and mass transfer. Energy & Fuels, 2011, 25(12): 5802–5809
|
| 20 |
Karousos D S, Labropoulos A I, Sapalidis A, Kanellopoulos N K, Iliev B, Schubert T J S, Romanos G E. Nanoporous ceramic supported ionic liquid membranes for CO2 and SO2 removal from flue gas. Chemical Engineering Journal, 2017, 313: 777–790
|
| 21 |
Li K, Yu H, Qi G, Feron P, Tade M, Yu J, Wang S. Rate-based modelling of combined SO2 removal and NH3 recycling integrated with an aqueous NH3-based CO2 capture process. Applied Energy, 2015, 148: 66–77
|
| 22 |
Qi G, Wang S. Thermodynamic modeling of NH3-CO2-SO2-K2SO4–H2O system for combined CO2 and SO2 capture using aqueous NH3. Applied Energy, 2017, 191: 549–558
|
| 23 |
Manovic V, Anthony E J, Loncarevic D. SO2 Retention by CaO-based sorbent spent in CO2 looping cycles. Industrial & Engineering Chemistry Research, 2009, 48(14): 6627–6632
|
| 24 |
Li Y, Liu C, Sun R, Liu H, Wu S, Lu C. Sequential SO2/CO2 capture of calcium-based solid waste from the paper industry in the calcium looping process. Industrial & Engineering Chemistry Research, 2012, 51(49): 16042–16048
|
| 25 |
Wappel D, Joswig S, Khan A A, Smith K H, Kentish S E, Shallcross D C, Stevens G W. The solubility of sulfur dioxide and carbon dioxide in an aqueous solution of potassium carbonate. International Journal of Greenhouse Gas Control, 2011, 5(6): 1454–1459
|
| 26 |
Ebrahimi S, Picioreanu C, Kleerebezem R, Heijnen J J, van Loosdrecht M C M. Rate-based modelling of SO2 absorption into aqueous NaHCO3/Na2CO3 solutions accompanied by the desorption of CO2. Chemical Engineering Science, 2003, 58(16): 3589–3600
|
| 27 |
Vidal B F, Ollero P, Gutiérrez Ortiz F J, Villanueva A. Catalytic seawater flue gas desulfurization process: An experimental pilot plant study. Environmental Science & Technology, 2007, 41(20): 7114–7119
|
| 28 |
Al-Anezi K, Hilal N. Effect of carbon dioxide in seawater on desalination: A comprehensive review. Separation and Purification Reviews, 2006, 35(3): 223–247
|
| 29 |
Smithson G L, Bakhshi N N. Kinetics and mechanism of carbonation of magnesium oxide slurries. Industrial & Engineering Chemistry Process Design and Development, 1973, 12(1): 99–106
|
| 30 |
Liu Q, Maroto-Valer M M. Studies of pH buffer systems to promote carbonate formation for CO2 sequestration in brines. Fuel Processing Technology, 2012, 98(16): 6–13
|
| 31 |
Swanson E J, Fricker K J, Sun M, Park A H. Directed precipitation of hydrated and anhydrous magnesium carbonates for carbon storage. Physical Chemistry Chemical Physics, 2014, 16(42): 23440–23450
|
| 32 |
Hosseini T, Daneshpayeh M, Selomulya C, Haque N, Zhang L. Chemical kinetic modeling and parameter sensitivity analysis for the carbonation of Ca2+ and Mg2+ under ambient conditions. Hydrometallurgy, 2017, 167: 141–152
|
| 33 |
Zhao Y, Wu M, Guo X, Zhang Y, Ji Z, Wang J, Liu J, Liu J, Wang Z, Chi Q, Yuan J. Thorough conversion of CO2 through two-step accelerated mineral carbonation in the MgCl2-CaCl2-H2O system. Separation and Purification Technology, 2019, 210: 343–354
|
| 34 |
Shen Z, Chen X, Tong M, Guo S, Ni M, Lu J. Studies on magnesium-based wet flue gas desulfurization process with oxidation inhibition of the byproduct. Fuel, 2013, 105(1): 578–584
|
| 35 |
del Valle-Zermeño R, Formosa J, Aparicio J A, Chimenos J M. Reutilization of low-grade magnesium oxides for flue gas desulfurization during calcination of natural magnesite: A closed-loop process. Chemical Engineering Journal, 2014, 254(7): 63–72
|
| 36 |
Wang F, Dreisinger D, Jarvis M, Hitchins T. Kinetics and mechanism of mineral carbonation of olivine for CO2 sequestration. Minerals Engineering, 2019, 131: 185–197
|
| 37 |
Bobicki E, Liu Q X, Xu Z H, Zeng H B. Carbon capture and storage using alkaline industrial wastes. Progress in Energy and Combustion Science, 2012, 38(2): 302–320
|
| 38 |
Aloisi G. A pronounced fall in the CaCO3 saturation state and the total alkalinity of the surface ocean during the Mid Mesozoic. Chemical Geology, 2018, 487: 39–53
|
| 39 |
Al-Yaseri A Z, Lebedev M, Barifcani A, Iglauer S. Receding and advancing (CO2 + brine+ quartz) contact angles as a function of pressure, temperature, surface roughness, salt type and salinity. Journal of Chemical Thermodynamics, 2016, 93: 416–423
|
| 40 |
Teng H, Masutani S M, Kinoshita C M, Nihous G C. Solubility of CO2 in the ocean and its effect on CO2 dissolution. Energy Conversion and Management, 1996, 37(6–8): 1029–1038
|
| 41 |
Weiss R F. Carbon dioxide in water and seawater: The solubility of a non-ideal gas. Marine Chemistry, 1974, 2(3): 203–215
|
| 42 |
Feely R, Sabine C, Lee K, Berelson W, Kleypas J, Fabry V, Millero F. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science, 2004, 305(5682): 362–366
|
| 43 |
Park S, Lee M G, Park J. CO2 (carbon dioxide) fixation by applying new chemical absorption-precipitation methods. Energy, 2013, 59(11): 737–742
|
| 44 |
Yang J, Yu X H, Yan J Y, Tu S T, Dahlquist E. Effects of SO2 on CO2 capture using a hollow fiber membrane contactor. Applied Energy, 2013, 112: 755–764
|
| 45 |
Li X, Zhang L, Zheng Y, Zheng C. Effect of SO2 on CO2 Absorption in flue gas by ionic liquid 1-ethyl-3-methylimidazolium acetate. Industrial & Engineering Chemistry Research, 2015, 54(34): 8569–8578
|
/
| 〈 |
|
〉 |