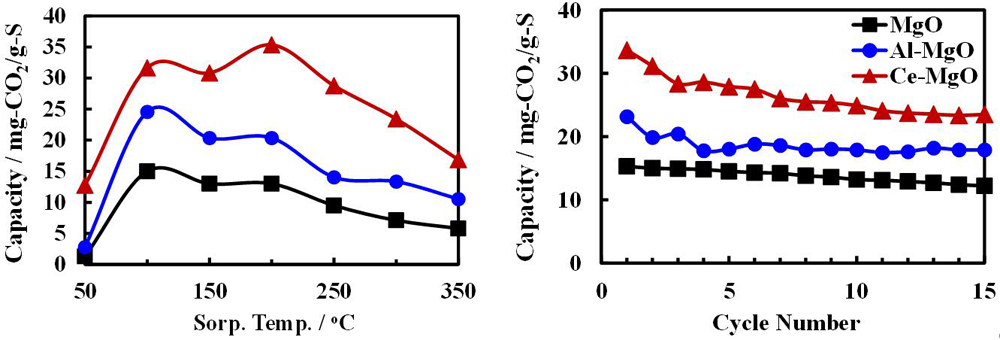
To improve the energy efficiency and reduce cost, many solid sorbents have been reported and these can be classified into four categories which are based on their operating temperatures: low (below room temperature), ambient (between room temperature and 100 °C), moderate (between 100 and 400 °C) and high (above 400 °C). Zeolites [
4,
12], metal-organic frameworks (MOFs) [
13,
14] and carbon materials [
15] are the typical adsorbents for the low temperature category, which is based on physisorption. Although a very high CO
2 capacity of 1320 mg-CO
2/g-S (S= sorbent) has been reported for MOF-5 (at –53 °C) [
16], the large reduction of CO
2 capacity at elevated temperatures and the negative impact of moisture greatly limits the application of these materials to CO
2 capture from flue gases. In the ambient-temperature category, amine-based solid sorbents [
2,
3,
17–
20] have received significant attention and are promising alternatives for CO
2 capture due to their high capacities (>100 mg-CO
2/g-S), high selectivities and good regenerabilities. Moisture even showed positive effect over these sorbents [
21]. In the high-temperature-adsorbent category, Li- [
22] and Ca-based [
23,
24] adsorbents have high CO
2 capacities although temperatures above 800 °C are normally required for their regeneration. Since the temperature of the flue gas vent is usually in the range of 150–400 °C [
25], the use of moderate temperature sorbents would seem to be the most suitable. Consequently, sorbents containing alkali or alkaline-earth metals such as Na
2CO
3, K
2CO
3, and MgO have attracted increasing attention since they can be operated at moderate temperatures (100–400 °C) and are applicable to both post- and pre-combustion capture [
26,
27].