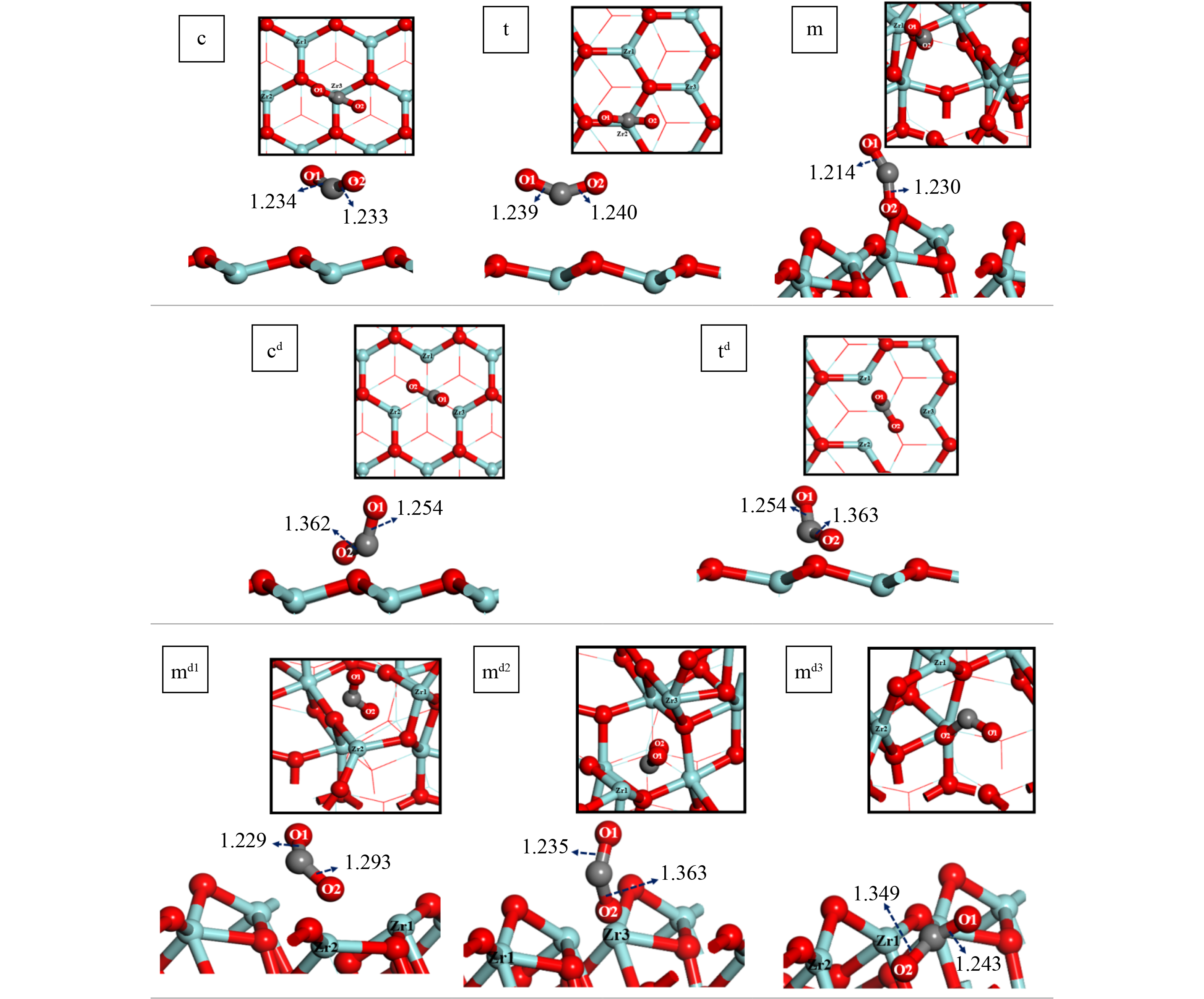
The supports of catalysts are generally prepared from metal oxides, and the commonly used supports are MgO, Al
2O
3, SiO
2, and ZrO
2. Among them, ZrO
2 has received a lot of attentions due to the presence of both acid-base centers and oxygen vacancies [
36,
37]. In 1993, Murota et al. [
38] first reported that ZrO
2 could promote the ability of CeO
2 to store oxygen and lower the temperature at which the reduction reaction occurs from 1100 to 900 K. In 2014, Chen et al. [
39] discovered that the higher the concentration of oxygen vacancies on the surface of ZrO
2 in the water–gas shift (WGS) reaction, the higher the catalytic efficiency of the catalyst. In 2017, Han et al. [
40] found that the presence of oxygen vacancies on the ZrO
2 surface improved the selectivity of CH
4 during the hydrogenation of CO to CH
4. In 2021, Petchmark et al. [
41] demonstrated that the ability of ZrO
2 to store hydrogen was increased in the presence of oxygen vacancies on its surface. These reports demonstrate that oxygen vacancies on the surface of ZrO
2 can improve its catalytic ability in various reactions. In fact, when ZrO
2 acts as a support, the oxygen vacancies can increase the contact area between the Ni particle and the support surface to keep the Ni particles well dispersed [
42]. The high dispersion, on the other hand, allows the Ni particles to remain in a small size, and the particle size can directly affect the performance of the catalyst [
43,
44]. Overall, ZrO
2 reduces the carbon build-up of the catalysts at high temperatures and increases the activity when it is used as a support.