1 Introduction
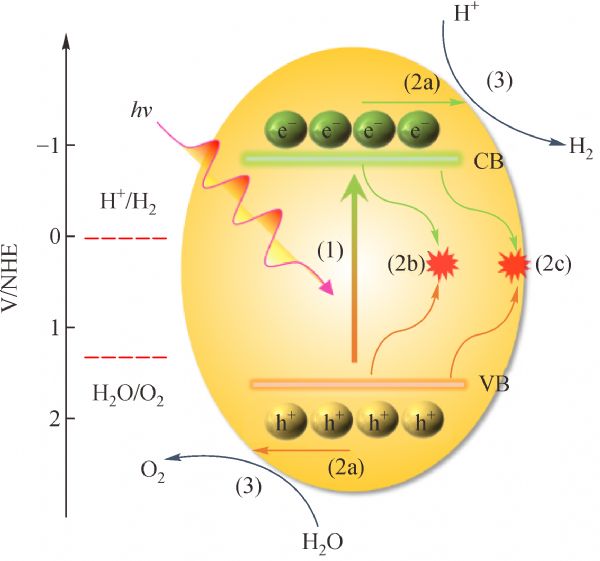
2 Basis of photocatalytic overall water splitting
3 Brief introduction to g-C3N4
3.1 g-C3N4
3.2 Basic physical and chemical properties
4 Recent progress on overall water splitting of CN based photocatalysts
4.1 Loading cocatalysts
4.1.1 Noble metals
4.1.2 Non-noble cocatalysts
Tab.1 Cocatalysts for photocatalytic overall water splitting over CN based photocatalysts |
| Cocatalyst | Light source | Efficiency | HER/(mmol·h−1) | H2/O2 | Mass/mg | Ref. |
|---|---|---|---|---|---|---|
| IrO2 | 300 W Xe lamp, λ>400 nm | AQY: 2.1% not mention the light wavelength | 45 | 2.25 | 30 | [34] |
| Pt, PtOx, and CoOx | 300 W Xe lamp, λ>300 nm | AQY: 0.3% at 405 nm | 12.2 | 1.94 | 200 | [35] |
| Pt and CoP | 300 W Xe lamp, λ>420 nm | NA | 2.1 | 2.10 | 80 | [36] |
| Pt and Co3O4 | 300 W Xe lamp, λ>300 nm | NA | 3.1 | 2.10 | 20 | [15] |
| Rh-RhOx | 300 W Xe lamp, λ>400 nm | AQY: 0.1% at 420 nm | 1.4 | 2.30 | 50 | [37] |
| PtMOx and Co3O4 | 300 W Xe lamp, λ>420 nm | AQY: 4.9% at 420 nm | 2.38 | 1.99 | 50 | [38] |
| Pt/Ni(OH)2 and Pt | 300 W Xe lamp | AQY: 4.2% at 420 nm | 26.60 | 2.10 | 20 | [39] |
| Pt and Ni(OH)2 | 150 W Xe lamp, 200 nm<λ<2500 nm | AQY: 1.48% at 405 nm | 15.5 | 1.99 | 200 | [40] |
| Pt/Ni(OH)2/CN | 300 W Xe lamp | AQY: 1.8% at 420 nm | 4.257 | 2.12 | 10 | [41] |
| Pt-Au single-sites | 300 W Xe lamp | AQY: 3% at 420 nm | 8.55 | 2.00 | 30 | [48] |
| Pt and Ru | Not mention, λ>350 nm | NA | 2.49 | 2.18 | 50 | [49] |
| C dots | 300 W Xe lamp, λ>420 nm | AQY: 16% at 420 nmSTH: 2% | ~8.4 | 2.02 | 80 | [50] |
| C dots | 300 W Xe lamp, λ>420 nm | NA | 0.25 | 2.00 | 50 | [51] |
| Amorphous NiO | 300 W Xe lamp, 420 nm<λ<700 nm | NA | 1.41 | 1.99 | 50 | [52] |
| CoO | 300 W Xe lamp, 420 nm<λ<700 nm | NA | 0.46 | 2.19 | 80 | [53] |
| MnO2 | 300 W Xe lamp, λ>420 nm | AQY: 3.82% at 420 nm | 5.53 | 2.00 | 100 | [54] |
| CNT and MnO2 | 70 W metal halide lamp, 380 nm<λ<780 nm | NA | 122 | About 2.00 | 30 | [55] |
| Mn2Co2C at C and MoOOH | 300 W Xe lamp | AQY: 1.45% at 20 nm | 8.876 | 2.05 | 150 | [56] |
| Co1-phosphide single sites | 300 W Xe lamp, λ>300 nm | AQY: 2.2% at 500 nm | 8.206 | 2.00 | 20 | [57] |
| WC1–x | 300 W Xe lamp, λ>420 nm | AQY: 11.24% at 420 nm | 3.364 | 2.02 | 40 | [58] |
4.2 Microstructure regulation and modification
4.2.1 Nanostructure modulation
4.2.2 Crystallinity regulation
Tab.2 Nanosctructure modification of CN for photocatalytic overall water splitting |
| Sample | Light source | Efficiency | HER/(μmol·h−1) | H2/O2 | Mass/mg | Other cocatalysts | Ref. |
|---|---|---|---|---|---|---|---|
| 3D CNNS | 300 W Xe lamp, λ>420 nm | AQY: 1.4% at 420 nm | 5.07 | 2.07 | 50 | 1 wt% Pt 3 wt% IrO2 | [59] |
| 3D Sea-urchin-like CN | 300 W Xe lamp, λ>420 nm | AQY: 0.43% at 420 nm | 1.04 | 2.04 | 25 | 3 wt% Pt | [60] |
| Alkali etched CN | 300 W Xe lamp, λ>420 nm | NA | 9.34 | 2.03 | 10 | 2 wt% Pt CQDs | [61] |
| Laser exfoliated CN | 300 W Xe lamp, λ>420 nm | NA | 0.426 | 2.28 | 10 | 1.4 wt% Pt | [62] |
| (Cring)-CN | 300 W Xe lamp, λ>420 nm | AQY: 5% at 420 nm | 11.13 | 2.00 | 30 | about 3 wt % Pt | [63] |
| Cco-CN | 300 W Xe lamp | AQY: 5.28% at 400 nm | 15.9 | 2.08 | 30 | None | [64] |
| Benzene ring incorporated CN | 300 W Xe lamp, λ>450 nm | AQY: 2.1% at 450 nm | 7 | NA | 5 | 1 wt% Pt | [65] |
| Na-CN | 300 W Xe lamp, λ>420 nm | AQY: 1.45% at 420 nm STH: 0.28% | 31.5 | 2.07 | 100 | 1 wt% Pt | [66] |
| K/B co-doped CN | 300 W Xe lamp, λ>400 nm | NA | 1.18 | 2.03 | NA | None | [70] |
| PTI∙HCl | 300 W Xe lamp | AQY: 2.1% at 380 nm | about 65 | about 2.00 | 100 | 1% Pt 9 wt% CoOx | [75] |
| PTI-550 | 300 W Xe lamp | AQY: 8% at 365 nm | 189 | 2.08 | 120 | 1wt% Pt 0.5 wt% Co | [76] |
4.3 Constructing nanocomposites
4.3.1 Type II
4.3.2 Z-scheme
4.3.3 p-n junction
Tab.3 CN based heterostructured photocatalysts for overall water splitting |
| Sample | Conditions | Efficiency | HER/(μmol·h−1) | H2/O2 | Mass/mg | Other cocatalysts | Ref. |
|---|---|---|---|---|---|---|---|
| CoO/CN | LED, λ>400 nm | NA | 2.51 | 1.81 | 50 | None | [77] |
| CoO Nanorod/CN | 300 W Xe lamp, λ>400 nm | AQY: 2.9% not mention the light wavelength | 54 | 2.16 | 30 | 3 wt% Pt | [78] |
| 2D/2D Co3(PO4)2/CN | 300 W Xe lamp, λ>400 nm | AQY: 1.32% at 420 nm | 18.78 | 2.12 | 50 | None | [79] |
| LaOCl-Coupled CN | 300 W Xe lamp | AQY: 0.4% at 380 nm | 22.3 | 2.08 | 50 | 0.5 wt% Pt and 0.2 wt% CoOx | [80] |
| P25/CN | Xe lamp | AQY: 0.96% at 350 nm | 3.742 | About 2.00 | 10 | 2 wt% Pt | [81] |
| MnOx/CN/TiO2/Au | 300 W Xe lamp, λ>420 nm | NA | 4.8 | 2.04 | 100 | None | [82] |
| CdS/Ni2P/CN | 300 W Xe lamp, λ>420 nm | NA | 0.778 | 2.01 | 50 | None | [83] |
| MnOx/CN/CdS/Pt | 300 W Xe lamp | NA | 65.17 | 2.03 | 50 | None | [84] |
| MnOx/CN/CdS/Pt | 300 W Xe lamp, λ>400 nm | AQY: 3.39% at 400 nm | 9.244 | 2.01 | 10 | None | [85] |
| CdSe QDs/P-CN | 300 W Xe lamp, l>420 nm | NA | 5.65 | 2.04 | 50 | 1 wt% Pt | [86] |
| CN (3 wt% Pt) – NaI – WO3 (0.5 wt% Pt) | 300 W Xe lamp | NA | 22.2 | 2.00 | 300 | None | [87] |
| CN (3 wt% Pt) – NaI – WO3(0.5 wt% Pt) | 300 W Xe lamp, λ>395 nm | NA | 6.36 | 1.93 | 300 | None | [87] |
| CN/BiVO4, Fe2+/Fe3+ | 300 W Xe lamp | AQY: 1.8% at 420 nm | 81.6 | 2.02 | 50 | 2 wt% Pt | [88] |
| 2D α-Fe2O3/CN | 300 W Xe lamp, λ>400 nm | NA | 1.91 | 2.00 | 50 | 3 wt% Pt 0.1 wt% RuO2 | [89] |
| Mn doped Fe2O3/CN | 300 W Xe lamp, λ>400 nm | NA | 51 | 2.05 | 30 | 1 wt% Pt | [90] |
| Fe2O3/rGO/CN | 300 W Xe lamp, λ>400 nm | NA | 43.6 | 2.06 | 40 | Pt | [92] |
| CN@a-Fe2O3/Co-Pi | 300 W Xe lamp, λ>420 nm | NA | 0.196 | NA | 20 | None | [93] |
| a-Fe2O3@MnO2/CN | 300 W Xe lamp, λ>190 nm | NA | 124 | 2.07 | 30 | None | [94] |
| CN/BiFeO3 | 125 W Hg lamp with an UV filter | NA | 0.933 | 2.01 | 40 | None | [95] |
| Mn defective MnO2/Monolayer CN | 300 W Xe lamp, λ>400 nm | NA | 1.212 | 2.10 | 20 | 3 wt% Pt | [96] |
| WO3∙H2O/CN | 300 W Xe lamp, λ>420 nm | AQY: 6.2% at 420 nm | 48.2 | 2.07 | 100 | None | [97] |
| WO3/rGO/CN | 250 W halide lamp, λ>420 nm | AQY: 0.9% at 420 nm | 2.84 | 1.95 | 200 | 1 wt% Pt | [98] |
| BiVO4/CN | 300 W Xe lamp, λ>400 nm | NA | 15.6 | 2.14 | 100 | 3 wt% Pt | [99] |
| TiO2/CN/WO3/β-Ni(OH)2/ | 150W Xe lamp; NaI as redox mediator | AQY: 2.04% at 425 nm | 50.2 | 2.07 | 100 | 1 wt% Pt PtOx | [101] |
| CN p-n homojunction/Ti3C2 | 300 W Xe lamp, λ>420 nm | AQY: 8.7% at 350 nm | 6.271 | 2.05 | 10 | 3 wt% Pt | [108] |