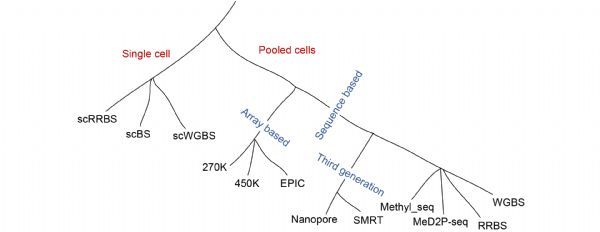
Bisulfite sequencing approach aims to investigate specific DNA sequences in single-base resolution. The treatment of genomic DNA with sodium bisulfite results in deamination of unmethylated cytosines to uracil, and leaves the methylated cytosines unaffected [
28]. Whole-genome bisulfite sequencing (WGBS) theoretically covers all the cytosine information, including low CpG density regions, such as the intergenic gene deserts and distal regulatory elements. It provides accurate binary calls of cytosine methylation status, and is considered as the golden standard method in the DNA methylation study, therefore, it has been widely used in the NIH Roadmap [
36] and ENCODE projects [
37]. WGBS is the most comprehensive one among the existing detection methods, while there are also two limits for the WGBS method that a considerable amount of DNA is required, and the data analysis is relatively cost and difficult. To investigate the methylome at a lower cost, the reduced representation bisulfite sequencing (RRBS) method was developed. It only sequence a fraction of the genome, which integrates
MspI restriction enzyme digestion, enrichment of CPG-rich regions, bisulfite conversion and the sequencing for the analysis of specific fragments [
38]. RRBS is less cost because it focuses on the enrichment of CpG-rich regions and is widely applied in methylation landscape of large-scale samples. The obvious drawback of RRBS is that it is limited to the loci containing
MspI cut sites, and it exhibits a lack of coverage at intergenic and distal regulatory elements that are relatively less studied.