1 Introduction
| Metal/Alloy | Melting point/°C | Metal/Alloy | Melting point/°C |
|---|---|---|---|
| Ga | 29.8 | Ga62.5In21.5Sn16 | 10.7 |
| Ga75.5In24.5 (EGaIn) | 15.4 | Ga62In25Sn13 | 11 |
| Ga86.5Sn13.5 | 20.5 | Ga67In29Zn4 | 13 |
| Ga88Sn12 | 25 | Ga72In12Zn16 | 17 |
| Ga96.1Zn3.9 | 24.7 | Ga61In25Sn13Zn1 | 8 |
| Ga97.6Al2.4 | 25.9 | Bi32.5In51Sn16.5 | 60 |
| Ga96.4Ag3.6 | 26.0 | Bi49In21Pb18Sn12 | 58 |
| Ga98.0Hg2.0 | 27.0 | Bi35In48.6Sn16Zn0.4 | 58.3 |
| Ga68.5In21.5Sn10 (Galinstan) | 13.2 | Bi44.7Pb22.6In19.1Sn8.3Cd5.3 | 47 |
2 Properties of liquid metal batteries
2.1 Development and properties of HTLMBs
| Advantages | Drawbacks |
|---|---|
| Excellent kinetics and transport properties | High temperature (>200°C) |
| Low cost | Low specific energy density (>200 W·h·kg−1) |
| Simple assembly | Low equilibrium cell voltages (<1.0 V) |
| Long lifespan | Static storage |
| Grid level energy storage potential | Rigorous thermal management |
| - | Stringent corrosion protection |
| - | Hermetic-seal demand |
2.2 Properties of gallium-based liquid metals
2.2.1 Liquidity
2.2.2 Metallicity
2.2.3 Safety
3 Gallium-based liquid metals in batteries
3.1 Gallium-based liquid metals as main reacting electrodes in batteries
3.1.1 Battery configurations
3.1.2 Working mechanisms
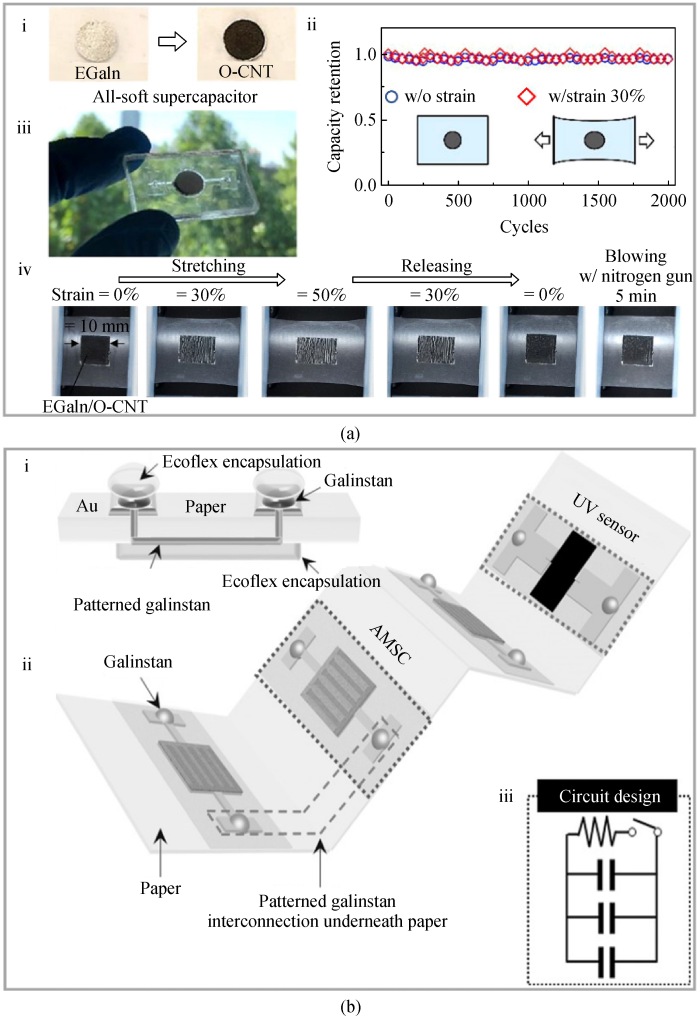
3.1.3 Battery functions
Tab.3 GBRTLMs as main reacting electrodes in batteries |
| Year | Anode material | Cathode material | Electrolyte | OCP/V | Main functionalities | Rechargeability | Ref. |
|---|---|---|---|---|---|---|---|
| 1963 | Ga | Porous silver | 6 mol/L KOH | 1.0 | – | No | [50] |
| 1963 | Ga | NiO | 6 mol/L KOH | 1.4 | – | Yes | [50] |
| 2017 | Ga | Conductive gel | 0.3 mol/L KOH | 1.1 | Flexible; 3D printing | No | [126] |
| 2018 | GaIn10 | Carbon fiber yarn@Pt | PAA-based KOH | 1.87 | Elastic; renewable anode; current controlled | No | [120] |
| 2019 | Ga75In25 | MnO2 | KOH/PAAm LiOH/KOH/PAAm | 1.47 | Stretchable | Yes | [123] |
| 2020 | Na54.1K45.9 | Ga78.6In21.4 Ga87.6Sn12.4 Ga68.5In21.5Sn10 | NaClO4/DME/FEC KPF6/DME/FEC | 1 | Facile fabrication; high safety | Yes | [76] |
| 2021 | Ga68.5In21.5Sn10 modified carbon fibers | Air electrodes | Filter paper (NaOH) | 1.12 | Flexibility; sensing; signal conversion | No | [51] |
| 2021 | Ga-foam | Carbon air electrode | Saturated NaCl | 1.35 | Lightweight; porous anode | No | [121] |
| 2021 | Ga68In22Sn10 | PANI | GaCl3/NH4Cl/PVA | 1.6 | Shape-variable | Yes | [127] |
Notes: OCP (open circuit potential); PAA (poly(acrylic acid)); PAAm (polyacrylamide); DME (dimethoxyethane); FEC (fluoroethylene carbonate); PVA (poly(vinyl alcohol)). |
3.2 Gallium-based liquid metals as auxiliary working electrodes in batteries
3.2.1 Lithium batteries
3.2.2 Non-lithium batteries
Tab.4 GBRTLMs as auxiliary working electrodes in batteries |
| Year | Battery type | Liquid metal | Methods | Functionalities | Results | Ref. |
|---|---|---|---|---|---|---|
| 2019 | LMB | GaInSnZn | Coated on current collector | Reduced nucleation barrier | Improve coulombic efficiency; reduce voltage fluctuations | [135] |
| 2020 | LMB | Ga | Dropped onto Li | Self-repairing LixGa layer | Long-term cycling life | [131] |
| 2020 | LMB | GaSn | Coated onto Li | Self-healing SEI layer | Superb rate capacity; long-term cycling life | [134] |
| 2020 | LMB | GaInSnZn | Coated on Mxene | Amorphous nucleation seeds | Improve coulombic efficiency | [136] |
| 2021 | LMB | GaInSnZn | Formed alloy with Li | Passivate Li metal surface | Superior electrochemical performance | [137] |
| 2008 | LIB | Ga | Confined in a carbon matrix | Self-healing | Buffer volume change | [100] |
| 2011 | LIB | Ga | Applied onto stainless steel | Self-healing | Higher capacity and higher durability of electrode | [75] |
| 2015 | LIB | Ga film | Applied onto stainless steel | Self-healing | Capacity decreased gradually | [101] |
| 2017 | LIB | Ga88Sn12 | Supported by carbon skeleton | Self-healing | Improve cycle life; deliver high capacity | [5] |
| 2018 | LIB | Galinstan | Embedded in N-rGO with Si | Heal the crack | High coulombic efficiency; better mechanical behavior | [150] |
| 2018 | LIB | Ga70In20Sn10 | Coated on Cu foil with Si | Spontaneous repairing | High capacity and stability; high coulombic efficiency | [143] |
| 2018 | LIB | Ga12.6Sn1.0 | Composited with Si | Self-healing; liquid buffer | High capacity; excellent cyclic performance | [151] |
| 2018 | LIB | Ga | Encapsulated by interwoven carbon fibers | Prevent the agglomeration | High capacity; high cycling stability; good rate performance | [152] |
| 2019 | LIB | Ga | Coated on Cu film | Self-healing | High capacity; better rate performance | [153] |
| 2019 | LIB | Ga88Sn12 | Coated with a carbon shell | Self-healing | Excellent capacity; stable cycling performance | [154] |
| 2019 | LIB | GaInSnZn | Confined in Mxene paper | Conductive substrate | Flexible and binder-free anode; high capacity and cycling | [155] |
| 2020 | LIB | Ga92Sn8 | Paired with polymer | Self-healing | Maintain mechanical integrity and better contact | [156] |
| 2020 | LIB | Galinstan | Introduced between Si/Cu | Self-healing | Avoid interfacial delamination; avoid early capacity decay | [157] |
| 2021 | LIB | Ga | In situ form | Self-healing | Improve the cycling stability | [158] |
| 2021 | ZIB | GaIn | Coating on zinc anode | Inward deposition | Ameliorate dendrite growth and electrode corrosion | [146] |
| 2020 | AlIB | Ga | Replace Al | Self-healing | Dendrite-free; corrosion-resistant; non-pulverization | [149] |
Notes: N-rGO (N-doped reduced graphene oxide). |