Introduction
Fossil fuel and alternative energy resources
Fuel cells
History of fuel cell and achievements
Tab.1 Summary of initial achievements in the field of fuel cells [21] |
| Scientists | Year | Achievements | Ref. |
|---|---|---|---|
| Nicolas and Carlisle | 1800 | Described the electrolysis of water | [21] |
| Grove | 1838 | Created the first gas battery | [22–24] |
| Monde and Langer | 1889 | Conducted experiments on hydrogen fuel cells | [22] |
| Ostwald | 1893 | Described theoretical performance of fuel cells | [22,23] |
| Jacquse | 1896 | Developed first fuel cell | [22] |
| Buar and Preis | 1921 | Experimented with high-temperature solid oxide electrodes | [22] |
| Bacon | 1939 | Researched alkaline fuel cells | [22] |
| DuPont, Parkersburg, West Virginia | 1950 | Teflon is used in membranes | [22,23,25] |
| Grubb | 1955 | Developed a sulfonated PEMFC | [22,23] |
| Brores and Ketelar | 1958 | Built a molten carbonate fuel cell | [22] |
| Central Technical Institute | 1959 | Researched SOFCs | [22] |
| IFC, Windsor Connecticut | 1960 | Developed a fuel cell power plant for the Apollo spacecraft | [22] |
| Elmore and Tanner | 1961 | Phosphoric acid fuel cell | [22] |
| IFC, Windsor Connecticut | 1970 | Oil crises, and developed a more powerful alkaline fuel cell for NASA’s space shuttle Orbiter | [22,25] |
| NASA jet propulsion | 1990 | First direct methanol fuel cell | [22,23] |
| Bauch up power | 2007 | Fuel cell being to be commercially sold as APU & stationary equipment’s power generation. | [22,25] |
| Honda | 2008 | Announced first mass production of fuel cell cars FCX clarity | [25] |
| Portable fuel cell chargers | 2009 | Residential micro fuel cell-CHP become commercially available in Japan | [25] |
How SOFCs works
Fuel cells types, applications, and advantages
Tab.2 Important advances in different kinds of fuel cells |
| Parameters | Type of fuel cell | |||||
|---|---|---|---|---|---|---|
| PEMFC | AFC | PAFC | MCFC | SOFC | Ref. | |
| Electrolyte | Hydrated polymeric ion exchange membranes | Mobilised or immobilized potassium hydroxide in asbestos matrix | Immobilised liquid phosphoric acid in SiC | Immobilised liquid molten carbonate in LiAlO2 | Perovskites (ceramics) | [25,31] |
| Electrodes | Carbon | Transition metals | Carbon | Nickel and nickel oxide | Perovskite and perovskite/metal cermet | [25,32] |
| Catalyst | Platinum | Platinum | Platinum | Electrode material | Electrode material | [33–36] |
| Interconnect | Carbon or metal | Metal | Graphite | Stainless steel or nickel | Nickel, ceramic, or steel | [25,37] |
| Operating temperature/°C | 40–80 | 65–220 | 205 | 650 | 600–1000 | [25,31] |
| Charge carrier | H+ | OH- | H+ | CO3 = | O = | [25,38] |
| External reformer for hydrocarbon fuels | Yes | Yes | Yes | No, for some fuels | No, for some fuels and cell designs | [25,39] |
| External shift conversion of CO to hydrogen | Yes+ purification to remove trace CO | Yes+ purification to remove CO and CO2 | Yes | No | No | [38,40] |
| Prime cell components | Carbon-based | Carbon-based | Graphite-based | Stainless-based | Ceramic | [25,31] |
| Product water management | Evaporative | Evaporative | Evaporative | Gaseous product | Gaseous product | [25,31] |
| Product heat management | Process gas+ liquid cooling medium | Process gas+ electrolyte circulation | Process gas+ liquid cooling medium or steam generation | Internal reforming+ process gas | Internal reforming+ process gas | [25,31] |
Geometrical design of SOFCs
Tab.3 Fabrication methods for the various types of fuel cell concepts and their components[44] |
| Design | Fabrication method | ||
|---|---|---|---|
| Electrolyte | Electrodes | Interconnect | |
| Tubular concept | CVD/EVD, plasma spraying | Slurry coating, plasma spraying, CVD/EVD | EVD, plasma spraying |
| Monolithic | Calender rolling, laminating, co-sintering | Calender rolling, laminating, co-sintering | Calender rolling, laminating, co-sintering |
| Planar | Tape casting, calender rolling | Screen printing, slurry coating | Ceramic or metal processing |
| Roller | Tape casting/co-sintering | Tape casting/co-sintering | Tape casting/co-sintering |
Planar design
Monolithic design
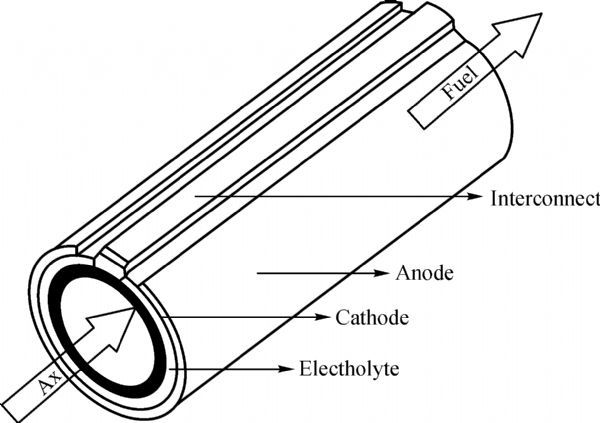
Tubular design
SOFC roll design
Material components of SOFCs
Anode
Tab.4 Conductivities of materials developed as anodes for SOFCs |
| Materials | DC conductivity/(S∙cm-1) | Advantage/disadvantage | Ref. |
|---|---|---|---|
| Sc0.1Y0.1Zr0.6Ti0.2O1.9 | 0.14 | Operate at high temperature | [51] |
| La0.8Sr0.2Fe0.8Cr0.2O3 | 0.5 | Low conductivity | [52] |
| La0.8Sr0.2Cr0.95Ru0.05O3 | 0.6 | Expensive | [8,53] |
| (La0.7Sr0.3)1–xCexCr1–xNixO3 | 5.03 | Carbon deposition | [54] |
| Sr0.88Y0.08TiO3 | 64 | High operating temperature | [55] |
| CrTi2O5 | 177 | Expensive | [8,56] |
| Ni-YSZ | 250 | High operating temperature | [57] |
| Ti0.34Nb0.66O2 | 340 | Very expensive | [58] |
| LaSrTiO2 | 360 | No compatibility | [59] |
| Ni-SDC | 573 | Coke formation | [8,60] |
| Ni-GDC | 1070 | Coke formation, and electronic performance degradation | [8,61] |
| Cu-CeO2 | 5200 | Improved electronic conductivity | [8,62] |
| Cu-GDCCrTi2O5 | 8500 | Good thermal expansion, and electronic performance | [8,63] |
Electrolyte and interconnects
Cathode
Tab.5 Conductivities of materials developed as a cathode for SOFCs [70] |
| Composition | TEC × 10-6/k-1 | T/°C | se/(S∙cm-1) |
|---|---|---|---|
| La0.8Sr0.2MnO3 | 11.8 | 900 | 300 |
| La0.7Sr0.3MnO3 | 11.7 | 800 | 240 |
| La0.6Sr0.4MnO3 | 13 | 800 | 130 |
| Pr0.6Sr0.4MnO3 | 12 | 950 | 220 |
| La0.8Sr0.2CoO3 | 19.1 | 800 | 1220 |
| La0.6Sr0.4CoO3 | 20.5 | 800 | 1600 |
| La0.8Sr0.2FeO3 | 12.2 | 750 | 150 |
| La0.5Sr0.5FeO3 | - | 550 | 352 |
| - | 800 | 369 | |
| La0.6Sr0.4FeO3 | 16.3 | 800 | 129 |
| Pr0.5Sr0.2FeO3 | 13.2 | 550 | 300 |
| Pr0.8Sr0.2FeO3 | 12.1 | 800 | 78 |
| La0.7Sr0.3Fe0.8Ni0.2O3 | 13.7 | 750 | 290 |
| La0.8Sr0.2Co0.2Fe0.8O3 | 20.1 | 600 | 1050 |
| La0.8Sr0.2Co0.2Ni0.8O3 | 15.4 | 600 | 125 |
| La0.8Sr0.2Co0.2Mn0.2O3 | 18.1 | 500 | 1400 |
| La0.6Sr0.4Co0.8Fe0.2O3 | 21.4 | 800 | 269 |
| La0.6Sr0.4Co0.2Fe0.8O3 | 15.3 | 600 | 330 |
| La0.4Sr0.6Co0.2Fe0.8O3 | 16.8 | 600 | |
| La0.8Sr0.2Co0.2Fe0.8O3 | 14.8 | 800 | 87 |
| La0.2Sr0.8Co0.8Fe0.2O3 | 19.3 | 800 | 1000 |
| La0.6Sr0.4Co0.9Fe0.1O3 | 19.2 | 700 | 1400 |
| Pr0.8Sr0.3Co0.2Fe0.8O3 | 12.8 | 800 | 76 |
| Pr0.7Sr0.3Co0.2Fe0.8O3 | 11.1 | 800 | 200 |
| Pr0.6Sr0.4Co0.8Fe0.2O3 | 19.69 | 550 | 950 |
| Pr0.4Sr0.6Co0.8Fe0.2O3 | 21.33 | 550 | 600 |
| Pr0.7Sr0.3Co0.9Fe0.1O3 | - | 700 | 1236 |
| Ba0.5Sr0.5Co0.8Fe0.2O3 | 20 | 500 | 30 |
| Sm0.5Sr0.5CoO3 | 20.5 | 700-900 | >1000 |
| LaNi0.6Fe0.4O3 | 11.4 | 800 | 580 |
| Sr0.9Ce0.1Fe0.8Ni0.2O3 | 18.9 | 800 | 87 |
SOFCs material structures
Tab.6 Common ceramic structural materials [81] |
| Structure | Lattice | Chemical formula |
|---|---|---|
| Caesiumchloride | SC | AX |
| Rock salt | FCC | AX |
| Fluorite | FCC | AX2 |
| Silicates | FCC | AX2 |
| Corundum | Hexagonal | A2X3 |
| Perovskites | SC | ABX3-A2B2X6 |
| Spinel | FCC | AB2X4 |
| Diamond | FCC | |
| Graphite | Hexagonal |
Perovkites structures
Single perovkites
Doubel perovskites
SOFCs trend from macro to nano-structured level
Tab.7 Some materials used in micro-scale SOFCs |
| Anode | Cathode | Electrolyte | Substrate | Temperature/°C | Ref. |
|---|---|---|---|---|---|
| Pt | Pt | 8YSZ | Foturan, silicon wafer | 450–550 | [103] |
| Ni | LSCF | GDC | - | 450–550 | [113] |
| Ni | LSM | 8YSZ | - | 400–700 | [114] |
| Pt | Pt, LSCF | 8YSZ | Foturan, glass-ceramic | 400–600 | [115] |
| Pt | Pt | 8YSZ | Silicon wafer, SiO2 | 500 | [116] |
| Pt | Pt | 8YSZ | Silicon wafer, Si3N4 | 350–400 | [117] |
| Pt | Pt | 8YSZ, CGO | Silicon wafer, Si3N4 | 350 | [118] |
| Ru | Pt | 8YSZ | Silicon wafer, Si3N4 | 265–350 | [119] |
| Pt | Pt | 8YSZ | Silicon wafer, Si3N4 | 400–450 | [120] |
| Ni | Pt, LSCF | CGO | Ni plate | 450 | [121] |
| Ni | Pt | 8YSZ | Porous Ni | 370–400 | [122] |
| Ni+ SDC | BSCF+ SDC | SDC | - | 500–600 | [123] |
Tab.8 Some materials used in nano-scale SOFCs |
| Anode | Cathode | Electrolyte | Substrate | Temperature/°C | Ref. |
|---|---|---|---|---|---|
| Ni | - | GDC(LiNa)C3 | - | 450–550 | [127] |
| Ni | LSM-YSZ | ScSZ | - | 700 | [128] |
| - | LSCF-GDC | GDC | - | 650–850 | [129] |
| Pt | LSCF | YSZ | Silicon wafer, Si3N4 | 450–500 | [130] |
| Ni-SDC | SSC | ScSZ | - | 600–700 | [131] |
| Ru | Pt | CGO-YSZ | - | 470–520 | [132] |
| Pt | Pt | YSZ | 350–500 | [133] | |
| Ni | Pt | YSZ | - | 600 | [134] |
SOFCs performance and efficiency
SOFCs power optimization in engineering applications
SOFCs research progress
Tab.9 Essential aspects with the basic parameters of any SOFC device |
| SOFCs parameters | Merits/strength | Limitation/weakness | Opportunity/availability | Threat/handling |
|---|---|---|---|---|
| Cost | * | * | ||
| Efficiency | * | |||
| Power density | * | |||
| Fuel utilization | * | * | ||
| Degradation rate | * | |||
| Materials | * | * | * | * |
| Design | * | * | * | * |
| Manufacturability | * | * | ||
| Durability | * | * | * | |
| Environmental impact | * | * | * | |
| Modularity | * | * | ||
| Scalability | * | |||
| Economic entitlement | * | * | ||
| Applications | * | * | * | * |
| Transportation and storage | * | * | ||
| Technological developments | * | * | * | |
| Life time | * | * |